Improving lung cancer diagnosis and patient management through genomic innovation
The critical need for lung cancer early detection
Lung cancer is the leading cause of death worldwide, and it is well established that early detection and diagnosis can reduce mortality.
~15M
The number of people who are eligible for annual screening in the U.S.1
1.6M
The number of lung nodules discovered incidentally each year.2
4.5%
The percentage of high-risk people in the U.S. who get screened for lung cancer.3
Pioneering new solutions to inform the lung cancer care continuum
Traditional Approach
- Low lung cancer screening rates3
- Uncertain nodule risk assessments
- Unnecessary invasive procedures
- Delayed diagnosis and treatment
Veracyte Approach
- Non-invasive genomic classifier
- Objective risk classification
- Earlier risk identification
- Precision insights
Solving cancer challenges
The future of lung cancer risk assessment lies in objective, non-invasive genomic testing that can help:
- Improve risk assessment
- Reduce unnecessary procedures
- Ensure patient gets the right treatment at the right time
How Lung Cancer Risk Assessment Works
A combination of powerful technologies:
1
Field of injury captured via simple nasal brush
Nasal epithelial cells are collected using a soft cytology brush. Using the well-studied foundational field of injury principle, we can investigate gene expression changes in individuals with a history of smoking. These changes can provide insights into the risk of developing lung cancer in patients with pulmonary nodules.2,3
2
Whole-transcriptome sequencing
This process examines the complete genetic expression profile (RNA) of nasal epithelial cells, providing deeper insights into cellular changes associated with lung cancer development in patients with a smoking history – including those who currently smoke or have a history of smoking.
3
Machine learning algorithms analyze complex genomic patterns
These algorithms deliver highly accurate risk assessment results by combining expression data with clinical factors to classify lung cancer risk in patients with pulmonary nodules and a history of smoking more than 100 cigarettes in their lifetime.4
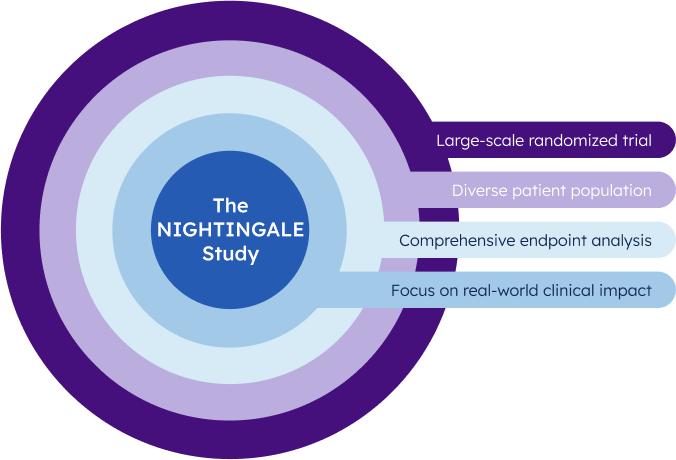
SETTING NEW STANDARDS IN CLINICAL EVIDENCE
What is the NIGHTINGALE study?
It is a landmark clinical utility study at hospitals and clinics around the United States to demonstrate the Percepta Nasal Swab test’s ability to improve patient care and outcomes.
Keep exploring our clinical and educational resources
REFERENCES
American Lung Association. New Report: Critically Low Lung Cancer Screening Rates Reveal Opportunity to Save More Lives. Published November 15, 2022. Accessed August 1, 2023. https://www.lung.org/media/press-releases/state-of-lung-cancer-2022
Gould MK, et al. Recent trends in the identification of incidental pulmonary nodules. Am J Respir Crit Care Med. 2015;192(10):1208-1214. https://doi.org/10.1164/rccm.201505-0990OC
American Lung Association. State of Lung Cancer 2023 Report. American Lung Association; 2023. Accessed October 25, 2024. https://www.lung.org/getmedia/186786b6-18c3-46a9-a7e7-810f3ce4deda/SOLC-2023-Print-Report.pdf
Lamb CR, Rieger-Christ KM, Reddy C, et al. A nasal swab classifier to evaluate the probability of lung cancer in patients with pulmonary nodules. Chest. 2024;165(4):1009-1019. https://doi.org/10.1016/j.chest.2023.11.036
This webpage contains forward-looking statements. These forward-looking statements involve risks and uncertainties. For more information, read our forward-looking statement.
This website contains information on products that are targeted to a wide range of audiences and could contain product details or information otherwise not accessible or valid in your country.
Please be aware that we do not take any responsibility for you accessing such information that may not comply with any legal process, regulation, registration or usage in the country of your origin.






