In Pipeline

Evolving Lung Nodule Risk Assessment
for Lung Cancer
Advancing precision in lung cancer risk assessment. Percepta Nasal Swab uses a simple, non-invasive, nasal brush and is more convenient than a blood draw.
- Risk Assessment
- Diagnosis
- Recurrence Monitoring
Percepta Nasal Swab test
What is the Percepta Nasal Swab test?
A non-invasive genomic test for lung nodule assessment in patients with a smoking history
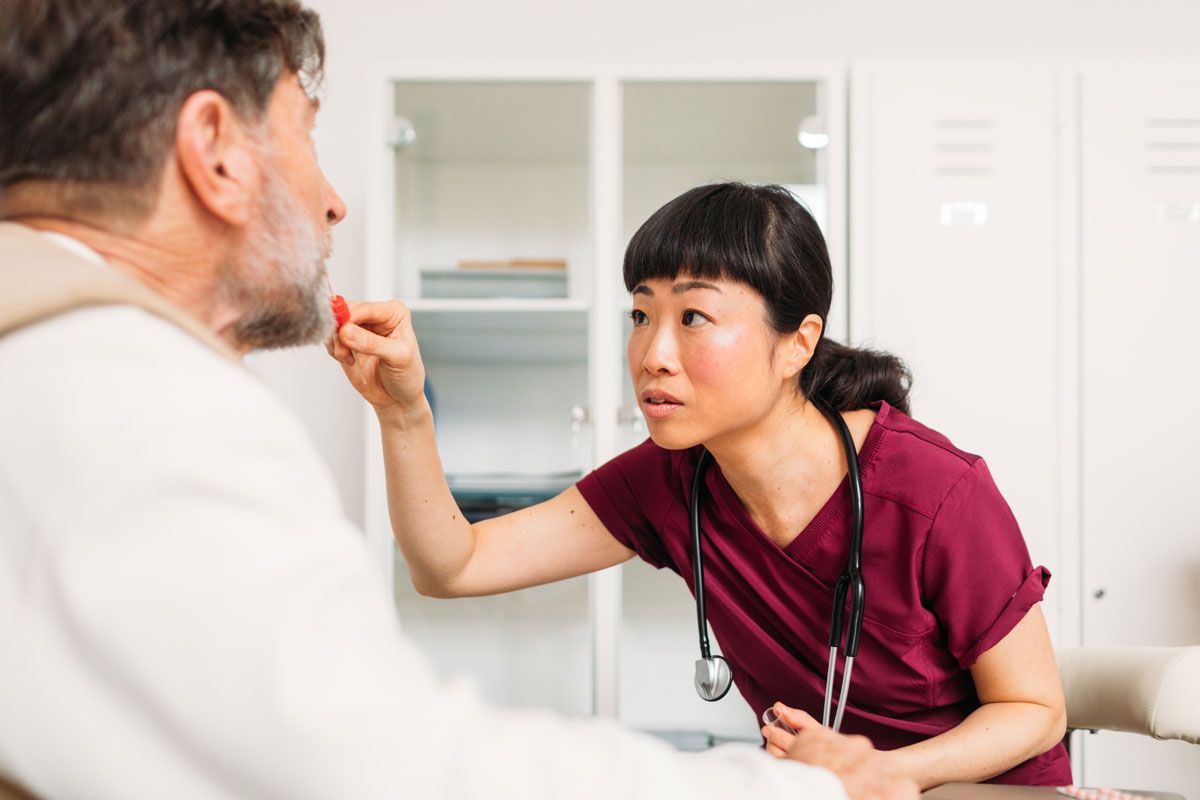
Percepta Nasal Swab is a cutting-edge, evidence-based test designed for patients with lung nodules and a history of smoking. Developed using whole-transcriptome sequencing and advanced machine learning, it leverages “field of injury” science to detect genomic changes linked to lung cancer in nasal epithelial cells.

Clinical validation data: evidence-based genomic assessment of lung nodules
The Percepta® Nasal Swab test’s clinical validation study, published in the journal CHEST, demonstrates significant advances in objective lung nodule risk assessment. This peer-reviewed research validates the test’s capability to support clinical decision-making for patients with lung nodules identified through CT screening.
Physicians need an objective, accurate tool to help guide care for patients when a lung nodule is found on a CT scan. Our findings demonstrate the Percepta Nasal Swab test's ability to enhance lung nodule risk assessment, potentially reducing unnecessary diagnostic procedures for low-risk patients while supporting timely intervention for those at high risk.”
Key Clinical Evidence
Consistent performance across nodule sizes and patient populations1
Low-Risk Patients
Negative predictive value (NPV) of 98%
In a population with a 25% cancer prevalence, Percepta Nasal Swab has a 97% sensitivity in identifying low-risk patients. This can help physicians and patients avoid unnecessary procedures.
High-Risk Nodules
Positive predictive value (PPV) of 70%
In a population with a cancer prevalence of 25%, Percepta Nasal Swab has a 92% specificity in identifying high-risk patients. This can help direct physicians to explore further procedures to obtain an accurate diagnosis.
Why are lung nodule evaluations important?
Lung nodules are an early indicator of lung cancer and are typically found using computed tomography (CT) scans.
1.6M
The number of lung nodules detected annually in the U.S.2
44%
The percentage of low-risk patients who undergo unnecessary invasive procedures3
50%
The percentage of lung cancer patients experience delayed diagnosis due to multiple biopsies4
Better Decisions Through Innovation
Why Veracyte is advancing lung nodule risk assessment
We aim to provide clinicians with a simple, non-invasive genomic test that transforms pulmonary nodule evaluation, so they can provide better care for their “at-risk” patients by helping to:
- Safely avoid unnecessary invasive procedures for patients
- Provide objective risk classification
- Reduce unnecessary procedures
- Support timely intervention for high-risk patients
- Enable confident clinical decision-making
The Percepta Nasal Swab test was developed with whole-transcriptome sequencing and machine-learning technology. It utilizes foundational “field of injury” science, which evaluates gene expression changes associated with lung cancer that can be found in epithelial cells in the nasal passages of current and former smokers.5,6
How the test works
Percepta Nasal Swab Test
1
Step 1: Sample collection
- Simple, in-office nasal brushing
- Non-invasive sample collection
2
Step 2: Testing & advanced analysis
- Whole-transcriptome sequencing
- Machine learning classification
- Novel genomic classifier
3
Step 3: Test results & risk classification
- Actionable results and evidence-based guidance
- Clear results for risk stratification
*Risk Stratification
 Low risk:
Low risk:Monitor with low dose CT
 Moderate risk:
Moderate risk:Consider non-surgical tissue sampling inclusive of bronchoscopy
 High risk:
High risk:Consider direct surgery and/or treatment
Next Step
Paving the way for Percepta
The next critical step for Percepta Nasal Swab is finalizing our clinical utility study, NIGHTINGALE, which will support reimbursement.
Keep exploring our clinical and educational resources.
REFERENCES
Lamb CR, Rieger-Christ KM, Reddy C, et al. A nasal swab classifier to evaluate the probability of lung cancer in patients with pulmonary nodules. Chest. 2024;165(4):1009-1019. https://doi.org/10.1016/j.chest.2023.11.036
Gould MK, et al. Recent trends in the identification of incidental pulmonary nodules. Am J Respir Crit Care Med. 2015;192(10):1208-1214. https://doi.org/10.1164/rccm.201505-0990OC
Tanner NT, et al. Management of pulmonary nodules by community pulmonologists: a multicenter observational study. Chest. 2015;148(6):1405-1414. https://doi.org/10.1378/chest.15-0630
Zhang Y, et al. Biopsy frequency and complications among lung cancer patients in the United States. Lung Cancer Manag. 2020;9(4):LMT40. https://doi.org/10.2217/lmt-2020-0022
Silvestri GA, et al. A bronchial genomic classifier for the diagnostic evaluation of lung cancer. N Engl J Med. 2015;373(3):243-251. https://doi.org/10.1056/NEJMoa1504601
Spira A, et al. Airway epithelial gene expression in the diagnostic evaluation of smokers with suspect lung cancer. Nat Med. 2007;13(3):361-366. https://doi.org/10.1038/nm1556
This webpage contains forward-looking statements. These forward-looking statements involve risks and uncertainties. For more information, read our forward-looking statement.
This website contains information on products that are targeted to a wide range of audiences and could contain product details or information otherwise not accessible or valid in your country.
Please be aware that we do not take any responsibility for you accessing such information that may not comply with any legal process, regulation, registration or usage in the country of your origin.









